Genetic testing in advanced or metastatic tumours
The molecular biomarkers and pathways involved in UC are key to understanding its biological heterogeneity and identifying specific subtypes, which may be used to predict treatment outcomes.A focus on the specific molecular subtypes in UC could provide the details you might need to personalise care for your patients. Indeed, it's already been seen that understanding the genomic alterations that can drive tumour growth has improved patient outcomes across cancer types.

International guidelines recommend the routine use of molecular testing in advanced or metastatic tumours, owing to its potential benefits for oncology patient care
- The ESMO guidelines recommend the routine use of NGS in non-squamous non-squamus non-small cell lung, prostate, ovarian and cholangiocarcinoma advanced cancers.
- The NCCN® Bladder Cancer Panel recommends that molecular/genomic testing, including for FGFR alterations, be performed ideally upon diagnosis of LA/mUC, to inform treatment decision-making and navigate through later lines of treatment.
- Notwithstanding, in UC, molecular biomarkers are not commonly used in routine clinical practice.

Optimised molecular testing protocols could enable the timely identification of relevant genetic alterations that could be driving your patients’ tumour growth

Both NGS and real-time PCR are widely used technologies to detect genetic alterations in cancerous tissues
While both qRT-PCR and NGS may use FFPE UC tissue samples and are highly sensitive and reliable, these techniques have some key differences:
- qRT-PCR has limited multiplex capacity, whereas NGS can screen hundreds of genomic loci
- qRT-PCR equipment is already placed in most laboratories, whereas NGS equipment is comparatively more technically demanding and expensive

Discuss available biomarkers and related molecular testing options with your pathologist today to better understand your patients’ tumour growth drivers
Value of MDTs in genetic testing
The management of UC is increasingly becoming multidisciplinary, with close cooperation and critical input needed from different specialities to inform effective disease management plans for patients.

When requesting molecular testing, make sure to consider the total turnaround time and liaise with your MDT to ensure you have the results to inform treatment decision-making and navigate through later lines of treatment

Work with your pathologist now to set up a testing infrastructure and/or ensure your testing capabilities cater to the advancement of biomarker-led precision medicine for your LA/mUC patients
- A pathologist is a vital partner when diagnosing UC and identifying the type of tumour present, which is critical to tailoring treatment plans for LA/mUC patients and achieving optimal clinical outcomes.
- As the turnaround time for different NGS platforms may range from ~2 hours to ~12 days, make sure to perform molecular testing upon diagnosis of advanced disease in order to inform treatment decision-making and develop a personalised treatment plan for your LA/mUC patients.
When establishing molecular testing protocols for your LA/mUC patients, it is important to liaise with the treating urologist to ensure sufficient sample quality is available
- Specimen quality, including tumour cellularity, preservation and fixation status, is critical for optimal molecular testing practices.
- FFPE specimens are normally used for most molecular testing techniques due to the advantage of preserved morphological details and the ability to store samples long term and at low cost.
- It is not always possible to acquire large quantities of DNA for genetic testing; however, NGS methods require nanogram quantities of DNA compared with traditional Sanger sequencing, which requires microgram quantities.


Discover what focusing on distinct molecular subtypes could mean for your LA/mUC patients
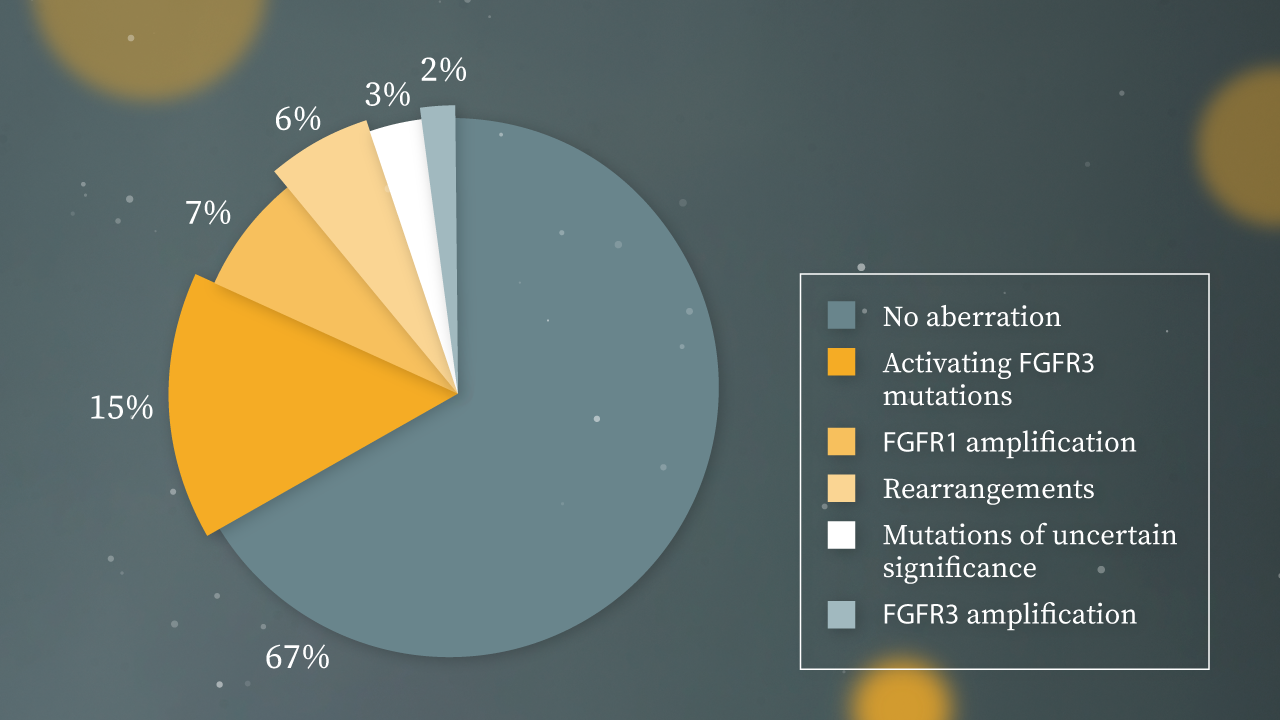
International guidelines (EAU and NCCN) recommend genetic testing to all mUC patients:

Explore more
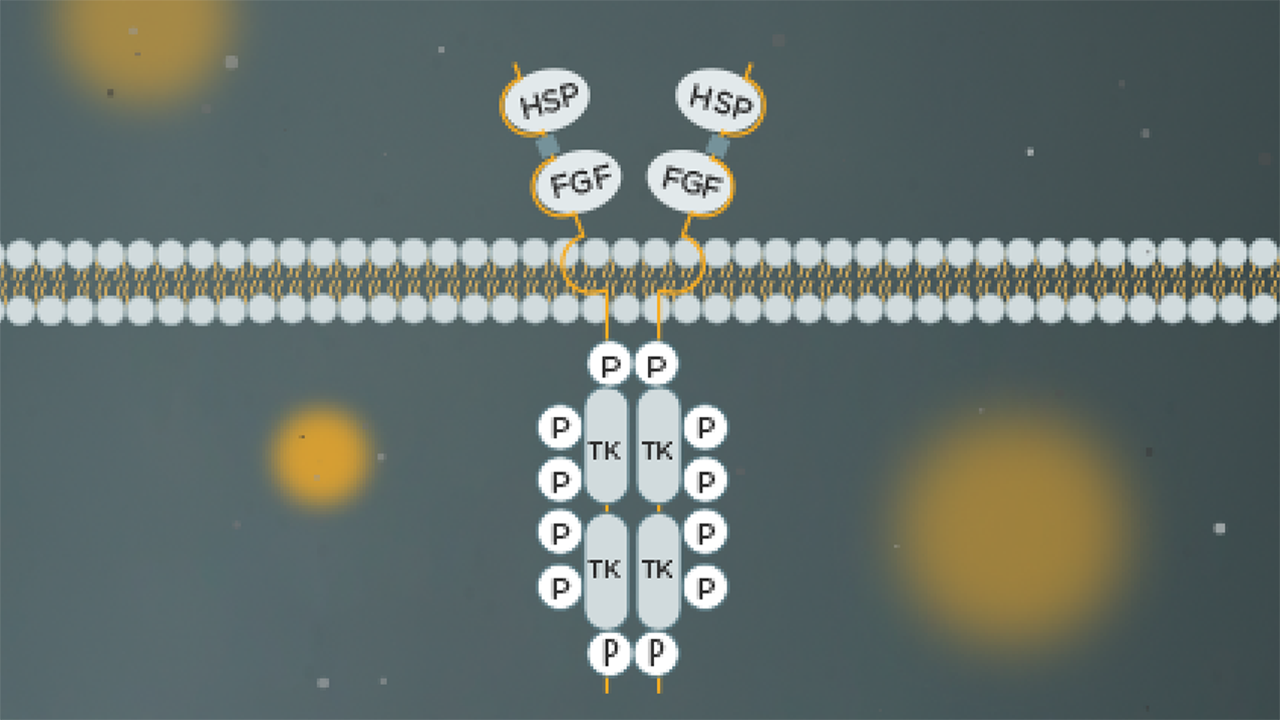
How do the prevalence and role of FGFR3 alterations drive disease for different tumour types?
What could the identification of molecular subtypes and distinct genetic disease drivers mean for the management of LA/mUC?
▼ Este medicamento está sujeito a monitorização adicional. Titular da Autorização de Introdução no Mercado: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Bélgica.
Para mais informações deverá contactar o Representante do Titular da Autorização de Introdução no Mercado: Janssen-Cilag Farmacêutica, Lda.
Medicamento de receita médica restrita, de utilização reservada a certos meios especializados.
RCM de Balversa®, veja aqui.
DNA: deoxyribonucleic acid; ESMO: European Society of Medical Oncology; FFPE: formalin-fixed paraffin-embedded; FGFR: fibroblast growth factor receptor; LA: locally advanced; MDT: multidisciplinary team; mUC: metastatic UC; NCCN®: National Comprehensive Cancer Network®; NGS: next-generation sequencing; PCR: polymerase chain reaction; qRT-PCR: quantitative real-time PCR; UC: urothelial carcinoma.