The role of FGFR3 alterations in LA/mUC
FGFR3 alterations are a prevalent potential driver in oncology, with continuous activation of the FGFR signalling pathway driving multiple tumourigenic processes across tumour types.As altered FGFR signalling is common in a wide variety of cancers, FGFR alterations represent an important potential target for treatment.
FGFR3 alterations are most commonly found in UC and have been identified across all grades and/or stages of bladder cancer
All in all, the molecular biomarkers and pathways involved in UC are key to understanding its biological heterogeneity and identifying specific subtypes, which may be used to predict treatment outcomes. Therefore, a focus on the distinct molecular subtypes in UC could help you see your individual LA/mUC patients more clearly.
Discuss available biomarkers and related molecular testing options with your pathologist today to better understand your patients’ tumour growth drivers
Explore more
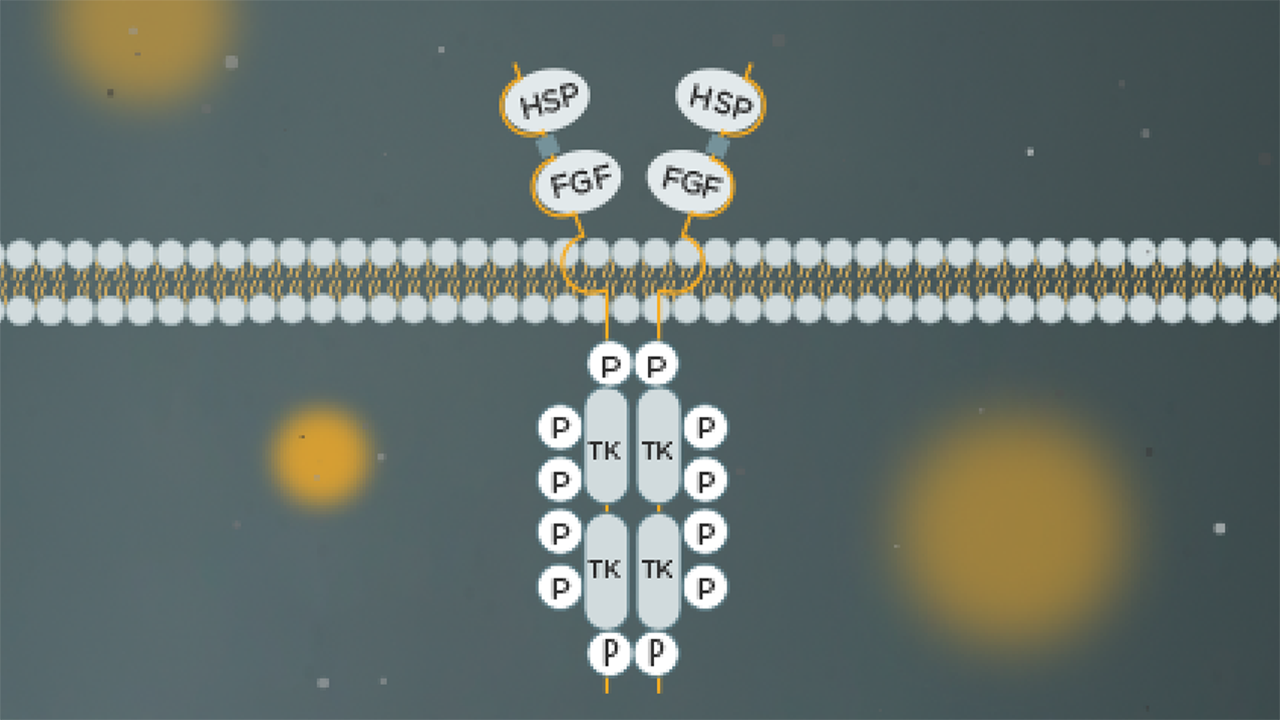
How do the prevalence and role of FGFR3 alterations drive disease for different tumour types?
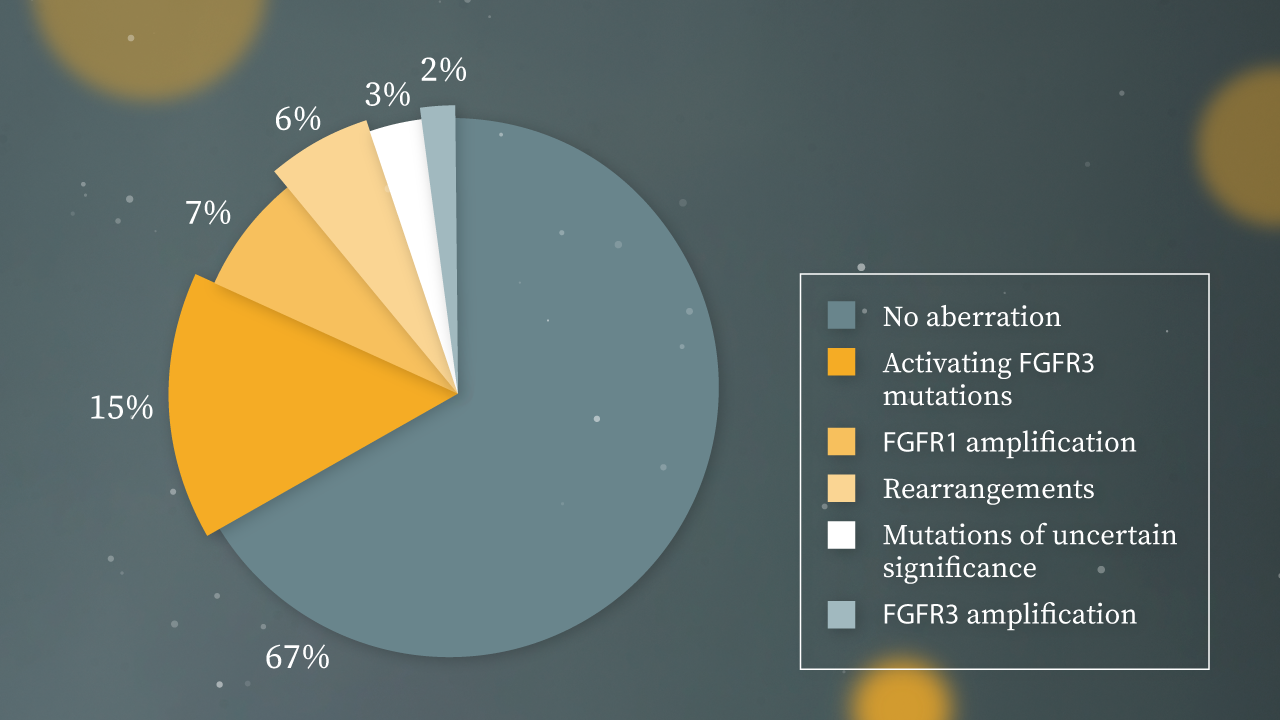
What could the identification of molecular subtypes and distinct genetic disease drivers mean for the management of LA/mUC?

How might optimised molecular testing protocols enable the timely identification of relevant genetic alterations that could be driving your patients’ tumour growth?
▼ Este medicamento está sujeito a monitorização adicional. Titular da Autorização de Introdução no Mercado: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Bélgica.
Para mais informações deverá contactar o Representante do Titular da Autorização de Introdução no Mercado: Janssen-Cilag Farmacêutica, Lda.
Medicamento de receita médica restrita, de utilização reservada a certos meios especializados.
RCM de Balversa®, veja aqui.
FGFR: fibroblast growth factor receptor; LA: locally advanced; mUC: metastatic UC; UC: urothelial carcinoma.